CSR activity
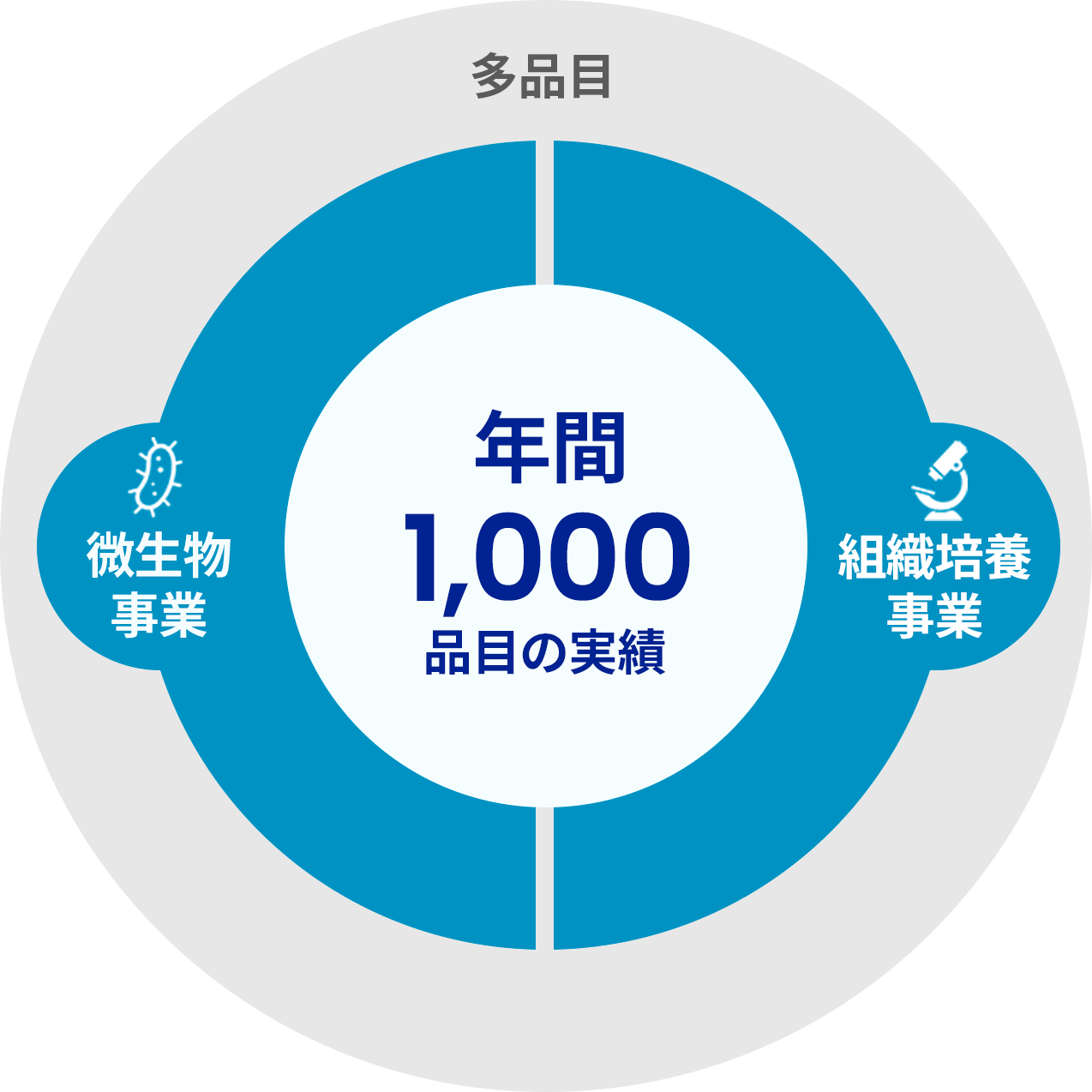
The Kohjin Bio Co., Ltd. brand has been built with a stable supply of a wide variety of products.
Unrivaled development power
Good at custom-designed products
Available even in small quantities
Stable supply
Rich track record
Own factory production/quality control
Quality first

ISO9001

ISO13485
- Acquired ISO13485
- Acquired ISO9001
- Acquired Manufacturing permission for in-Vitro Diagnostic Medicine
- Acquired Manufacturing permission for Poisonous and deleterious substances-manufacturing
- Acquired Cosmetics-manufacturing permission
- Acquired Cosmetics-manufacturing and sales permission
- Acquired Medical equipment manufacturing permission
- Acquired the third-class medical equipment manufacturing permission
- Acquired Medical equipment repairing permission
In order to achieve the above, we have established a quality management system (ISO9001 and ISO13485), regularly review it to maintain its effectiveness, and implement continuous improvements.
GMP compliant culture medium manufacturing factory
Culture medium manufacturing plant that complies with GMP (Good Manufacturing Practice) safety and quality control




Licensed facility for regenerative medicine
Kohjin Bio Co., Ltd. Saitama Cell Processing Center, which has received permission from the Ministry of Health, Labor and Welfare to manufacture specified cell products under the “Regenerative Medicine Safety Act,” processes cells from patient specimens received from medical institutions.
The inside of the cell processing facility is grade B, and the inside of the safety cabinet is grade A, ensuring an appropriate environment. With the access control system, entry and exit to the clean room is security controlled.
In addition, the environmental monitoring system monitors and records clean room environmental data such as temperature/humidity, differential pressure, and ventilation frequency, as well as temperature control equipment such as refrigerators, freezers, and CO2 incubators 24 hours a day.

